Murakami et al. Kidney Int. 2021 Jul;100(1):196-205.
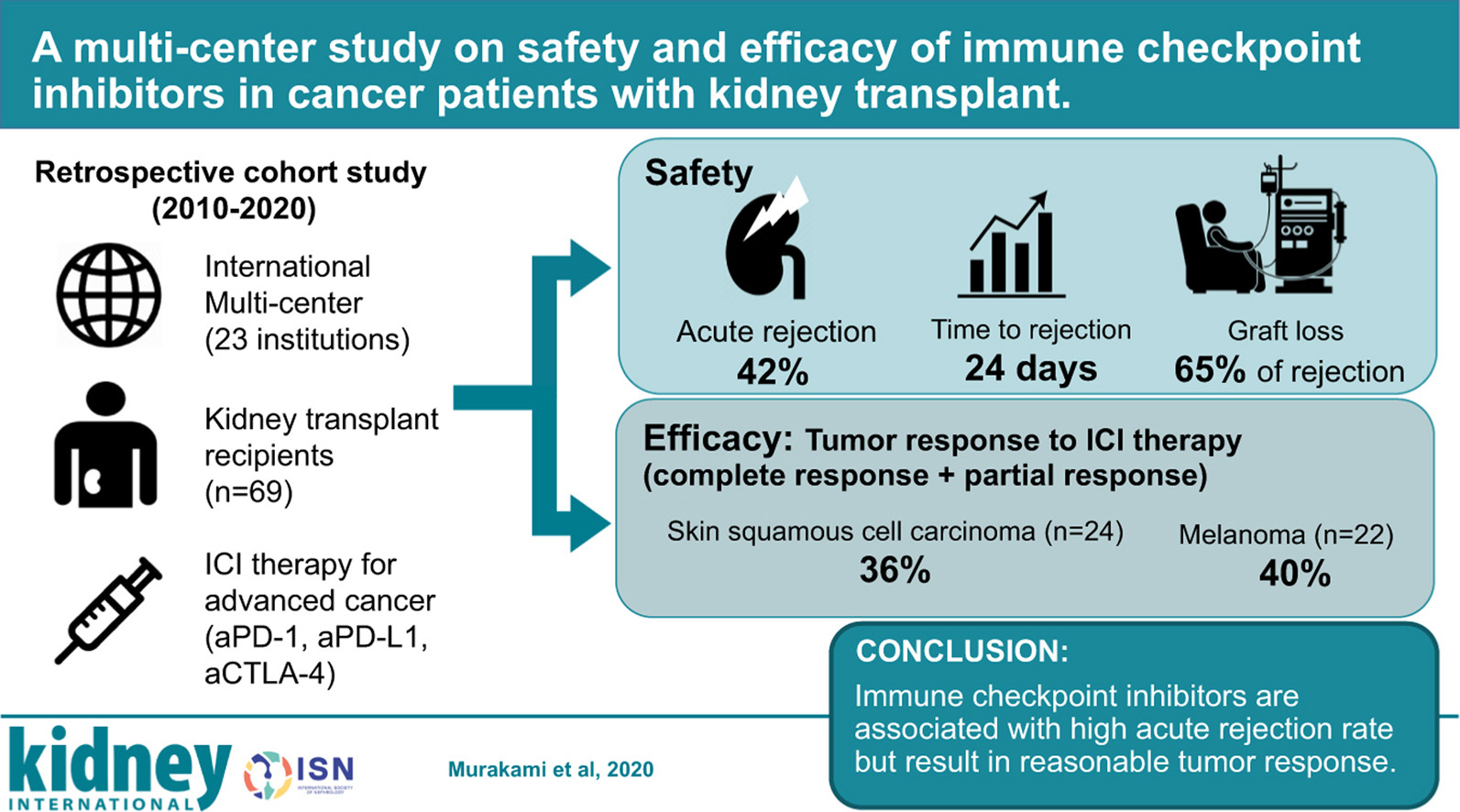
Background: Immune checkpoint inhibitors (ICIs) are widely used for various malignancies. However, their safety and efficacy in patients with a kidney transplant have not been defined. To delineate this, we conducted a multicenter retrospective study of 69 patients with a kidney transplant receiving ICIs between January 2010 and May 2020.
Methods: For safety, we assessed the incidence, timing, and risk factors of acute graft rejection. For efficacy, objective response rate and overall survival were assessed in cutaneous squamous cell carcinoma and melanoma, the most common cancers in our cohort, and compared with stage-matched 23 patients with squamous cell carcinoma and 14 with melanoma with a kidney transplant not receiving ICIs.
Results: Following ICI treatment, 29 out of 69 (42%) patients developed acute rejection, 19 of whom lost their allograft, compared with an acute rejection rate of 5.4% in the non-ICI cohort. Median time from ICI initiation to rejection was 24 days. Factors associated with a lower risk of rejection were mTOR inhibitor use (odds ratio 0.26; 95% confidence interval, 0.09-0.72) and triple-agent immunosuppression (0.67, 0.48-0.92). The objective response ratio was 36.4% and 40% in the squamous cell carcinoma and melanoma subgroups, respectively. In the squamous cell carcinoma subgroup, overall survival was significantly longer in patients treated with ICIs (median overall survival 19.8 months vs. 10.6 months), whereas in the melanoma subgroup, overall survival did not differ between groups.
Conclusions: ICIs were associated with a high risk of rejection in patients with kidney transplants but may lead to improved cancer outcomes. Prospective studies are needed to determine optimal immunosuppression strategies to improve patient outcomes.
2024
- Van Meerhaeghe T, Murakami N, Le Moine A, Brouard S, Sprangers B, Degauque N. Fine-tuning tumor- and allo-immunity: advances in the use of immune checkpoint inhibitors in kidney transplant recipients. Clin Kidney J. 2024;17(4):sfae061. PMID: 38606169.
- Murakami N, Wong G. Introduction: Clinical Innovations in Transplant Onconephrology. Semin Nephrol. 2024 Mar 11:151493. doi: 10.1016/j.semnephrol.2024.151493. Epub ahead of print. PMID: 38472079.
- Hanna GJ, Dharanesswaran H, Giobbie-Hurder A, Harran JJ, Liao Z, Pai L, Tchekmedyian V, Ruiz ES, Waldman AH, Schmults CD, Riella LV, Lizotte P, Paweletz CP, Chandraker AK, Murakami N*, Silk AW*. Cemiplimab for Kidney Transplant Recipients With Advanced Cutaneous Squamous Cell Carcinoma. J Clin Oncol. 2024;42(9):1021-1030. PMID: 38252908. (co-senior authors)
2023
- Mizuno H, Murakami N. Multi-omics Approach in Kidney Transplant: Lessons Learned from COVID-19 Pandemic. Curr Transplant Rep. 2023 Dec;10(4):173-187. PMID: 38152593.
- Terashita M, Selamet U, Midha S, Nadeem O, Laubach J, Rennke HG, Murakami N. Clinical Outcomes of Monoclonal Gammopathy of Renal Significance Without Detectable Clones. Kidney Int Rep. 2023 Sep 22;8(12):2765-2777. PMID: 38106576.
- Abdelrahim M, Esmail A, Abudayyeh A, Murakami N, Victor D, Kodali S, Cheah YL, Simon CJ, Noureddin M, Connor A, Saharia A, Moore LW, Heyne K, Kaseb AO, Gaber AO, Ghobrial RM. Transplant Oncology: An Emerging Discipline of Cancer Treatment. Cancers (Basel). 2023 Nov 9;15(22):5337. PMID: 38001597.
- Murakami N, Reich AJ, He K, Gelfand SL, Leiter RE, Sciacca K, Adler JT, Lu E, Ong SC, Concepcion BP, Singh N, Murad H, Anand P, Ramer SJ, Dadhania DM, Lentine KL, Lakin JR, Alhamad T. Kidney Transplant Clinicians’ Perceptions of Palliative Care for Patients With Failing Allografts in the US: A Mixed Methods Study. Am J Kidney Dis. 2023 Epub ahead of print. PMID: 37726050.
- Murakami N, Reich AJ, Pavlakis M, Lakin JR. Conservative Kidney Management in Kidney Transplant Populations. Semin. Nephrol. 43: 15141. PMID: 37499572
- DiToro D, Murakami N, Pillai S. T-B Collaboration in Autoimmunity, Infection, and Transplantation. Transplantation. 2023 Jun 14. PMID: 37314442
- Yeung MY*, Murakami N*, Kafetzi ML*, Simmons DP, Wood I, Macaskill P, Towle M, Della Gatta J, Stevens J, Comeau E, Baronas J, Mohsin N, Chen M, Lee JH, Lane WJ, Milford EL, Guleria I. Impact of allele-specific anti-HLA class I antibodies on organ allocation. Am J Transplant. 2023 May 29:S1600-6135(23)00487-2. PMID: 37257653. (*co-first authors)
- Blosser CD, Portuguese AJ, Santana C, Murakami N. Transplant Onconephrology: An Update. Semin Nephrol. 2023 May 18;42(6):151348. PMID: 37209580.
- Dunlap GS, DiToro D, Henderson J, Shah SI, Manos M, Severgnini M, Weins A, Guleria I, Ott PA, Murakami N*, Rao DA*. Clonal dynamics of alloreactive T cells in kidney allograft rejection after anti-PD-1 therapy. Nat Commun. 2023 Mar 21;14(1):1549. PMID: 36941274 (*co-senior authors)
- Abboud K, Umoru G, Esmail A, Abudayyeh A, Murakami N, Al-Shamsi HO, Javle M, Saharia A, Connor AA, Kodali S, Ghobrial RM, Abdelrahim M. Immune Checkpoint Inhibitors for Solid Tumors in the Adjuvant Setting: Current Progress, Future Directions, and Role in Transplant Oncology. Cancers (Basel). 2023 Feb 23;15(5):1433. PMID: 36900226.
- Murakami N, Borges TJ, Win TS, Abarzua P, Tasigiorgos S, Kollar B, Barrera V, Sui SH, Teague JE, Bueno E, Clark RA, Lian CG, Murphy GF, Pomahac B, Riella LV. Low-dose IL-2 promotes immune regulation in face transplantation: A pilot study. Am J Transplant. 2023 Feb 3:S1600-6135(23)00239-3. Epub ahead of print. PMID: 36740193.
- Murakami N, Hayden R, Hills T, Al-Samkari H, Casey J, Del Sorbo L, Lawler PR, Sise ME, Leaf DE. Therapeutic advances in COVID-19. Nat Rev Nephrol. 2023 Jan;19(1):38-52. PMID: 36253508.
2022
- Bharadwaj P, Shrestha S, Pongracz T, Concetta C, Sharma S, Le Moine A, de Haan N, Murakami N, Riella LV, Holovska V, Wuhrer M, Marchant A, Ackerman ME. Afucosylation of HLA-specific IgG1 as a potential predictor of antibody pathogenicity in kidney transplantation. Cell Rep Med. 2022 Nov 15;3(11):100818. PMID: 36384101.
- Gupta S, Garcia-Carro C, Prosek JM, Glezerman I, Herrmann SM, Garcia P, Abudayyeh A, Lumlertgul N, Malik AB, Loew S, Beckerman P, Renaghan AD, Carlos CA, Rashidi A, Mithani Z, Deshpande P, Rangarajan S, Shah CV, Seigneux S, Campedel L, Kitchlu A, Shin DS, Coppock G, Ortiz-Melo DI, Sprangers B, Aggarwal V, Benesova K, Wanchoo R, Murakami N, Cortazar FB, Reynolds KL, Sise ME, Soler MJ, Leaf DE; ICPi-AKI Consortium Investigators. Shorter versus longer corticosteroid duration and recurrent immune checkpoint inhibitor-associated AKI. J Immunother Cancer. 2022 Sep;10(9):e005646. PMID: 36137651.
- Kawashima S, Joachim K, Abdelrahim M, Abudayyeh A, Jhaveri KD, Murakami N. Immune checkpoint inhibitors for solid organ transplant recipients: clinical updates. Korean Journal of Transplantation. 2022; 36(2): 82-98. PMID: 35919193.
- Murakami N, Webber AB, Nair V. Transplant Onconephrology in Patients With Kidney Transplants. Adv Chronic Kidney Dis. 2022 Mar;29(2):188-200.e1. PMID: 35817526.
- Cron DC, Murakami N, Xiang L, Markmann JF, Yeh H, Adler JT. Anastomosis Time and Outcomes after Donation after Circulatory Death Kidney Transplantation. J Am Coll Surg. 2022 Jun 1;234(6):999-1008. PMID: 35703788.
- Ng JH, Izard S, Murakami N, Jhaveri KD, Sharma A, Nair V. Outcomes of kidney transplantation in patients with myeloma and amyloidosis in the US. Nephrol Dial Transplant. 2022 Jun 10:gfac196. PMID: 35687020.
- Schreiber B, Abdelrahim M, Abudayyeh A, Murakami N. Emerging Concepts in Managing Malignancy in Kidney Transplant Patients. Semin Nephrol. 2022 Jan;42(1):63-75. PMID: 35618396.
- Akiyama S, Hamdeh S, Murakami N, Cotter TG, Suzuki H, Tsuchiya K. Pregnancy and neonatal outcomes in women receiving calcineurin inhibitors: A systematic review and meta-analysis. Br J Clin Pharmacol. 2022 May 20. PMID: 35593302.
- Abdelrahim M, Esmail A, Saharia A, Abudayyeh A, Abdel-Wahab N, Diab A, Murakami N, Kaseb AO, Chang JC, Gaber AO, Ghobrial RM. Utilization of Immunotherapy for the Treatment of Hepatocellular Carcinoma in the Peri-Transplant Setting: Transplant Oncology View. Cancers (Basel). 2022 Mar 30;14(7):1760. PMID: 35406533; PMCID: PMC8997123.
- Gassen RB, Borges TJ, Pérez-Sáez MJ, Zhang H, Al Jurdi A, Llinàs-Mallol L, Aoyama B, Lima M, Pascual J, Sage PT, Murakami N, Riella LV. T cell depletion increases humoral response by favoring T follicular helper cells expansion. Am J Transplant. 2022 Mar 23. PMID: 35320600.
- Murakami N, Baggett ND, Schwarze ML, Ladin K, Courtwright AM, Goldberg HJ, Nolley EP, Jain N, Landzberg M, Wentlandt K, Lai JC, Shinall MC, Ufere NN, Jones CA, Lakin JR. Top Ten Tips Palliative Care Clinicians Should Know About Solid Organ Transplantation. J Palliat Med. 2022 May; PMID: 35275707.
- Murakami N*. Gelfand SL*, Sciacca KR, Kileen K, Leiter RE, Adler JT, Chandraker AK, Lakin JR. Inpatient Kidney Palliative Care for Kidney Transplant Recipients with Failing Allografts. Kidney Med. 2022; 4:100398. PMID: 35243310. PMC8861950.
2021
- Borges TJ, Murakami N, Lape IT, Gassen RB, Liu K, Cai S, Daccache J, Safa K, Shimizu T, Ohori S, Paterson AM, Cravedi P, Azzi J, Sage PT, Sharpe AH, Li XC, Riella LV. Overexpression of PD-1 on T cells Promotes Tolerance in Cardiac Transplantation via ICOS-Dependent Mechanism. JCI Insight. 2021 Dec 22;6(24):e142909. PMID: 34752418. PMC8783692.
- Gupta S, Short S.A.P, Sise ME, Prosek JM, Madhavan SM, Soler MJ, Ostermann M, Herrmann SM, Abudayyeh A, Anand S, Glezerman I, Motwani SS, Murakami N, Wanchoo R, Ortiz-Melo DI, Rashidi A, Sprangers B, Aggarwal V, Malik AB, Loew S, Carlos CA, Chang W, Beckerman P, Mithani Z, Shah CV, Renaghan AD, De Seigneux S, Campedel L, Kitchlu A, Shin DS, Rangarajan S, Deshpande P, Coppock G, Eijgelsheim M, Seethapathy HS, Lee M, Strohbehn IA, Owen DH, Husain M, García-Carro C, Bermejo S, Lumlertgul N, Seylanova N, Flanders L, Isik B, Mamlouk O, Lin JS, Garcia P, Kaghazchi A, Khanin Y, Kansal SK, Wauters E, Chandra S, Schmidt-Ott KM, Hsu RK, Tio MC, Mothi SS, Singh H, Schrag D, Jhaveri KD, Reynolds KL, Cortazar FB, Leaf DE. Acute Kidney Injury in Patients Treated with Immune Checkpoint Inhibitors. J Immunother Cancer. 2021 Oct;9(10):e003467. PMID: 34625513. PMC8496384.
- Adam BA, Murakami N, Reid G, Du K, Jasim R, Boils CL, Bu L, Hill PD, Murray AG, Renaudin K, Roufosse C, Weins A, Wen K, Riella LV, Mengel M. Gene Expression Profiling in Kidney Transplants With Immune Checkpoint Inhibitor-Associated Adverse Events. Clin J Am Soc Nephrol. 2021; 16:1376. PMID: 34244334. PMC8729568.
- Pérez-Sáez MJ, Uffing A, Leon J, Murakami N, Watanabe A, Borges TJ, Sabbisetti V, Cureton P, Kenyon V, Keating L, Yee K, Fernandes Satiro CA, Serena G, Hildebrandt F, Riella CV, Libermann TA, Wallace Wang M, Pascual J, Bonventre JV, Cravedi P, Fasano A, Riella LV. Immunological Impact of a Gluten-Free Dairy-Free Diet in Children with Kidney Disease. Frontiers Immunol. 2021; 12:624821. PMID: 34149688. PMC8208082.
- Win TS, Crisler WJ, Dyring-Andersen B, Lopdrup R, Teague JE, Zhan Q, Barrera V, Ho Sui S, Tasigiorgos S, Murakami N, Chandraker A, Tullius SG, Pomahac B, Riella LV, Clark RA. Immunoregulatory and Lipid Presentation Pathways Are Upregulated in Human Face Transplant Rejection. Journal of Clinical Investigation. 2021; 131(8):e135166. PMID: 33667197. PMC8262560.
- Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, Abdelrahim M, Khairallah P, Shirazian S, Kukla A, Owoyemi IO, Alhamad T, Husami S, Menon M, Santeusanio A, Blosser C, Zuniga SC, Soler MJ, Moreso F, Mithani Z, Ortiz-Melo D, Jaimes EA, Gutgarts V, Lum E, Danovitch GM, Cardarelli F, Drews RE, Bassil C, Swank JL, Westphal S, Mannon RB, Shirai K, Kitchlu A, Ong S, Machado SM, Mothi SS, Ott PA, Rahma O, Hodi FS, Sise ME, Gupta S, Leaf DE, Devoe CE, Wanchoo R, Nair VV, Schmults CD, Hanna GJ, Sprangers B, Riella LV, Jhaveri KD. A Multi-Center Study on Safety and Efficacy of Immune Checkpoint Inhibitors in Cancer Patients With Kidney Transplant. Kidney Int. 2021; 100(1): 196. PMID: 33359528. PMC8222056.
2020
- Danesh MJ, Mulvaney PM, Murakami N, Riella LV, Silk AW, Hanna GJ, Schmults CD. Impact of Corticosteroids on Allograft Protection in Renal Transplant Patients Receiving anti-PD-1 Immunotherapy. Cancer Immunol Immunother. 2020;69 (9):1937-1941. PMID: 32588077. PMC7479641.
- Cortazar FB, Kibbelelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik BA, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE. Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study. J Am Soc Nephrol. 2020;31(2):435-446. PMID: 31896554. PMC7003302.
2019
- Magee CN*, Murakami N*, Borges TJ, Shimizu T, Safa K, Ohori S, Cai S, Uffing A, Azzi J, Elyaman W, Charbonnier LM, Liu K, Toprak D, Visner G, Chatila TA, Siebel CW, Najafian N, Riella LV. Notch-1 Inhibition Promotes Immune Regulation in Transplantation Via Regularory T Cell-Dependent Mechanisms. 2019; 140:846-63. PMID: 31266349. PMC6722011.